A NATIONAL FULL-SERVICE HEALTHCARE LEADER
RCL was launched early-cycle in response to the worldwide COVID-19 pandemic. Our companies employ a professional team of doctors, business leaders, medical staff, laboratory technicians and software engineers.
The RCL companies were established to help global business’ understand and implement the most efficient, accurate and cost effective solutions to mitigate COVID-19 in the workplace.
RCL delivers results. Through our national network of high complexity laboratories, dedicated and trained staffing, proprietary software delivery systems, certified medical research team, and hands-on client-centric management, RCL does what most other companies cannot; we deliver results. Learn more
- PROVEN
- EXPERIENCED
- VETTED
PROTECTING FILM & TELEVISION PRODUCTIONS
NATIONWIDE
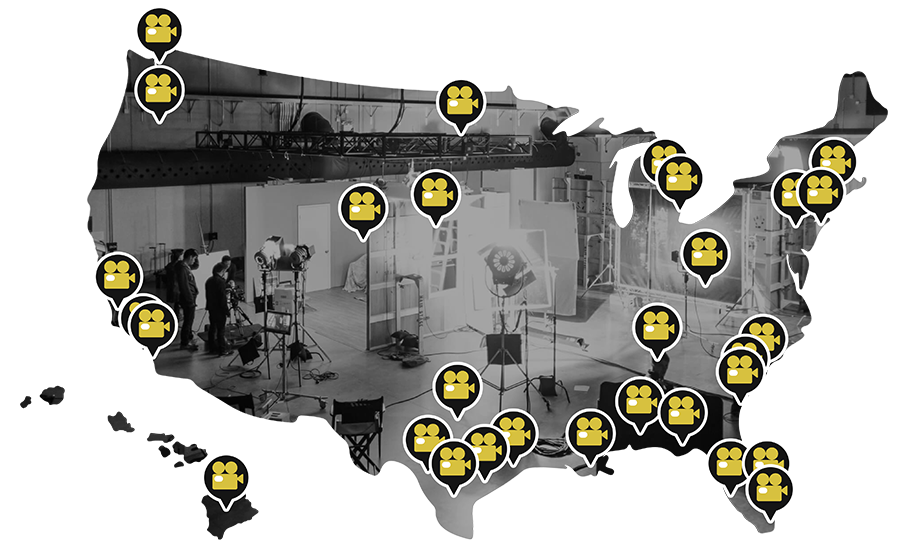
- NEW YORK, NY
- ATLANTA, GA
- CHICAGO, IL
- HONOLULU, HI
- WILMINGTON, NC
- CHARLOTTE, NC
- DENVER, CO
- AUSTIN, TX
- DALLAS, TX
- LOS ANGELES, CA
- ORANGE COUNTY, CA
- SAN FRANCISCO, CA
- RALEIGH, NC
- PORTLAND, OR
- JACKSON HOLE, WY
- STLOUIS, MO
- MIAMI, FL
- TAMPA, FL
- HOUSTON, TX
- PITTSBURGH, PA
- WASHINGTON DC
- MILWAUKEE, WI
- FORT LAUDERDALE, FL
- SEATTLE, WA
- BOZEMAN, MT
- OKLAHOMA CITY, OK
- CINCINNATI, OH
- NEW ORLEANS, LA
AVAILABLE ANYWHERE IN THE USA



